CME Accreditation
12th International Winter Arrhythmia School was approved for the following credits by Continuing Professional Development, Faculty of Medicine, University of Toronto:
Royal College of Physicians and Surgeons of Canada – 17.5 Section 1 credits
The American Medical Association – 17.5 AMA PRA Category 1 credits
The College of Family Physicians of Canada – 4.0 Mainpro-M1 credits
Through an agreement between the Royal College of Physicians and Surgeons of Canada and the American Medical Association, physicians may convert Royal College MOC credits to AMA PRA Category 1 Credits™. Information on the process to convert Royal College MOC credit to AMA credit can be found at www.ama assn.org/go/internationalcme.
12th International Winter Arrhythmia School was endorsed by:
Workshops
Building on the success of previous conferences, our workshops offered specialized instruction for nurses, EP technicians, residents, fellows, and allied professionals.
We deployed world-class faculty to engage in discussions and expect, as always, fierce educational argument.
International Winter Arrhythmia School Founder and Chair: Dr. Eugene Crystal
12th Annual Hosting Faculty
12th Annual International Faculty
|
12th Annual National Faculty
|
Organizers Banafsheh Arouny & Seddigheh Baktash
Supporting Organizations
We are grateful to the following partners for their generous support of last year’s International Winter Arrhythmia School:
12th International Winter Arrhythmia School is endorsed by:
2015 Sponsors:
Location
The 12th International Arrhythmia School was hosted at the Westin Trillium House at Blue Mountain in Collingwood, Ontario. Blue Mountain is a ski resort located approximately 90 minutes by car outside of Toronto. 220 Gord Canning Drive (Formerly Mountain Drive), Blue Mountains Ontario, L9Y 0V9, Canada Phone: (705) 443-8080
Abstracts
Presentation Downloads
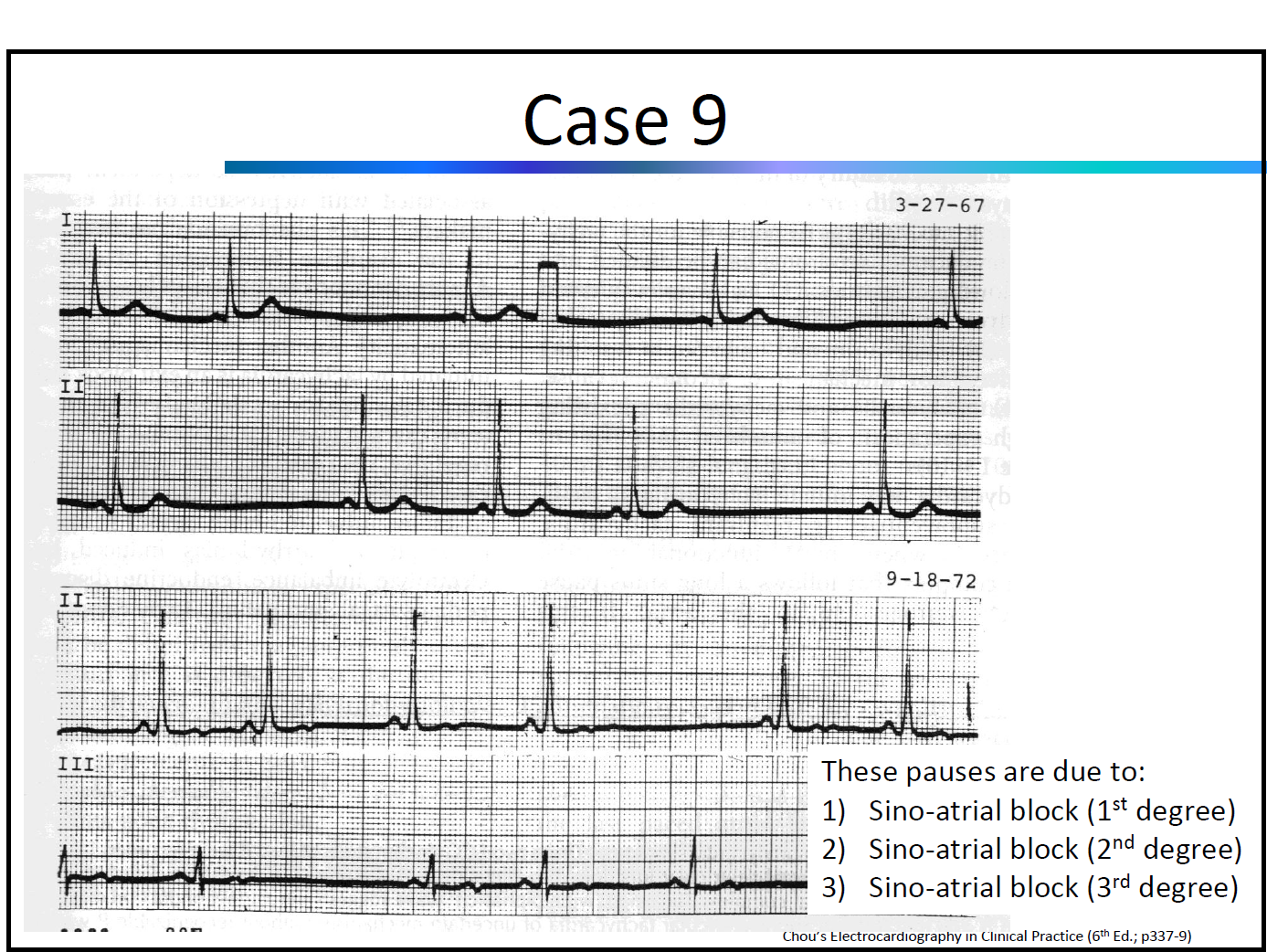
ECG Workshop by Dr. A. Baranchuk & Dr. K. Quinn
ECG in Cardiac Devices by Dr. A. Pinter
ICD Troubleshooting (case-based) by Dr. A. Pinter
Telemetry Workshop (level 1) by Dr. D. Kagal & Dr. I. Tiong
Telemetry Workshop (level 2) by Dr. D. Kagal & Dr. I. Tiong
Telemetry Workshop (level 3) by Dr. D. Kagal & Dr. I. Tiong
Intracardiac Echo – ICE, AcuNav by Dr. E. Camus
Managing Cardiac Devices at End of Life: Our Clinic’s Role in Proactive Care by
D. Campbell
Post Traumatic Stress in the Cardiac Patient by Dr. B. Baker
Post Implantable Defibrillator Shock Psychology Counselling by Dr. B. Baker
How to Become a Successful Researcher in Cardiology by Dr. M. Hartleib
Challenging Arrhythmia Cases by Dr. B. Makanjee
Pacemaker Troubleshooting (case-based) by Dr. D. Ng
Challenging Arrhythmia Cases by Dr. D. Ng
What is the Strategy of Ablation After STAR AF Trial by Dr. G. Amit
“Robotics in Ablation of Atrial Fibrillation” & “V-drive: Benefits and Challenges From User Perspective” by Dr. W. Spear
Indications for PVC Ablation by Dr. G. Nair
Duelling “PVI vs. Pace and Ablate” by Dr. G. Nair
Role of Stress Test in Arrhythmia Management by Dr. J. Healey
Magnet Application on “CIEDs” by Dr. D. Yung
Substrate Mapping Techniques and Technologies by Dr. D. Burkhardt
Robotics for Ischemic/structural VTs: Practical Tips and Tricks by Dr. D. Burkhardt
Post Implantation Wound Care by S. Turner
Duelling “Antiarrhythmic vs. Ablation of VT” by Dr. D. Burkhardt
Myths and Facts: A Discussion of MRI Imaging for Pacemaker and ICD Patients by M. Reitzel
Best Abstract Presenters
First Place went to Fariha Sadiq Ali for “Advanced Interatrial Block Predicts New Onset Atrial Fibrillation in Patients with Severe Heart Failure and Cardiac Resynchronization Therapy”.
Second Place went to Philippa Krahn for “Intrinsic Magnetic Resonance Characterization of Acute Ventricular RF Ablation Lesions”.
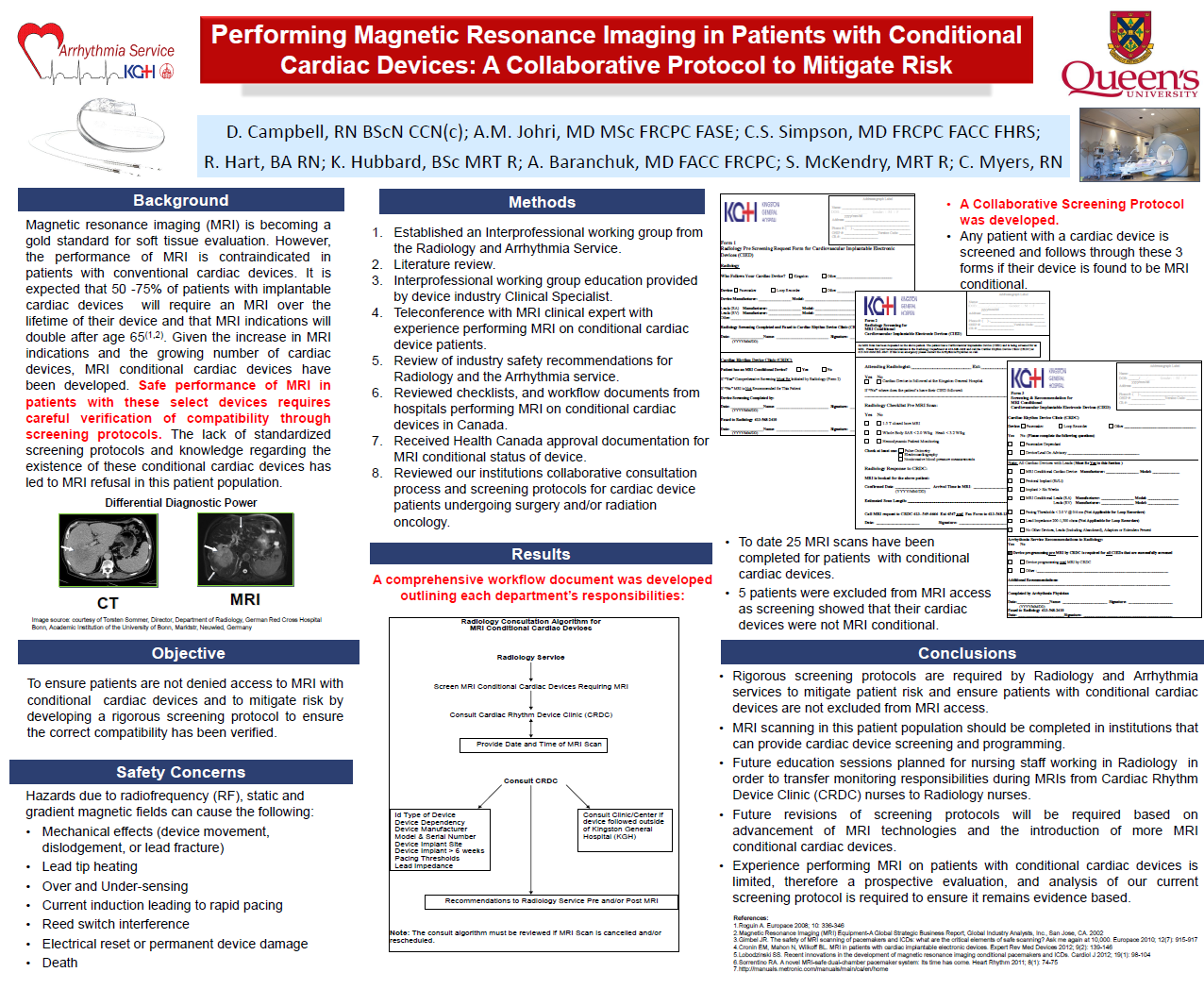
Third Place went to Debra Campbell for “Performing Magnetic Resonance Imaging in Patients with Conditional Cardiac Devices: A Collaborative Protocol to Mitigate Risk”.